While traditional opioids target the brain's MOP receptor for pain relief (causing euphoria), the NOP receptor can enhance pain relief while suppressing MOP's addictive side effects. Tris Pharma's new drug is a first-in-class molecule that equally targets both receptors, aiming for effective pain management without the addictive high.
Related Insights
The brain maintains balance by counteracting any deviation to the pleasure side with an equal and opposite reaction to the pain side. This opponent process is why we experience hangovers and why chronic indulgence leads to a dopamine deficit state, driving us to use more just to feel normal.
The company's strategy focuses on the critical period after short-acting analgesics (lasting 2-3 days) wear off, but before surgical pain (lasting 3-4 weeks) subsides. This gap is where opioid dependence often begins, creating a clear market opportunity for an extended-release, non-opioid solution.
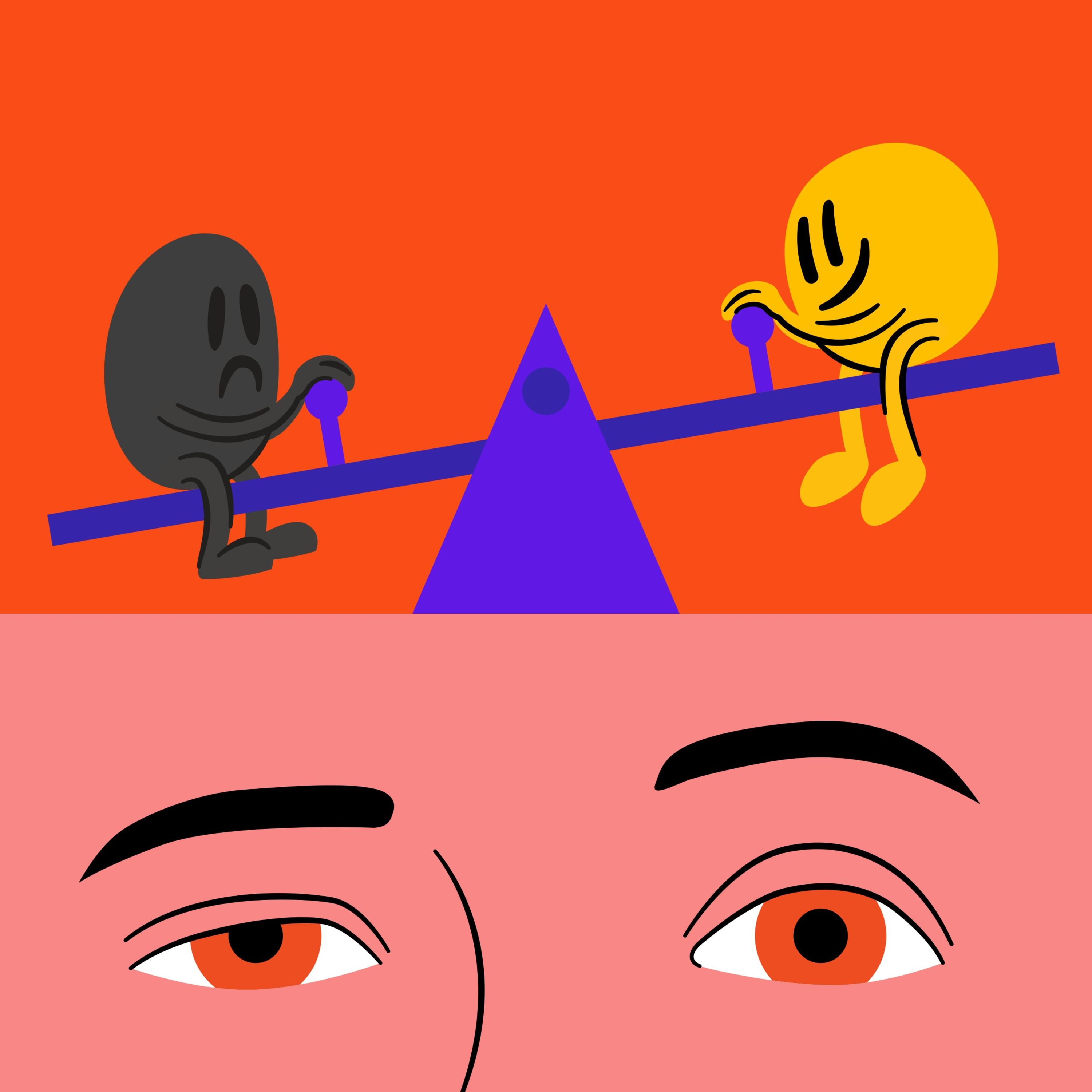
Neuroscience shows pleasure and pain are co-located in the brain and work like a seesaw. When we experience pleasure, the brain immediately compensates by tilting towards pain to restore balance. This neurological 'come down' is why constant pleasure-seeking eventually leads to a state of chronic pain and craving.
Many people use substances to treat anxiety or depression, not realizing the substance itself causes a dopamine deficit that mimics those conditions. Abstaining for four weeks allows the brain to reset its reward pathways and restore natural dopamine production, often resolving the symptoms entirely.
Up to 25% of people experience a euphoric response when taking opioids, a key driver of addiction. The risk is highest for the subset of this group (about 5-6% of the total population) who also have predisposed addictive tendencies. This shows how a prescribed medication can inadvertently lead to addiction in a vulnerable population segment.
Alley Therapeutics highlights a critical consequence of inadequate pain control: the transition from acute to chronic pain. By providing consistent relief during the crucial post-operative weeks, their product aims to prevent this long-term complication, which is associated with a nearly threefold higher risk in orthopedic surgery.
When comparing drugs with the same mechanism, like Alkermes' and Takeda's orexin agonists, a wider therapeutic index is a crucial differentiator. This superior safety-to-efficacy ratio allows for higher, more effective dosing without significant side effects, creating a competitive advantage and potential for broader market use.
The satiation signal from GLP-1s to the brain stem also down-regulates dopamine and the desire for it. This explains anecdotal reports and active studies on their effect in reducing cravings for nicotine, alcohol, shopping, and gambling.
Aardvark differentiates its drug from GLP-1s by claiming to abrogate hunger (a pain-avoidance drive from fasting), not just diminish appetite (a positive, reward-based drive). This novel mechanism targets the discomfort of food deprivation, offering a distinct approach in the crowded weight-loss market.
To get FDA approval, new opioids must undergo Human Abuse Potential (HAP) studies. In these counter-intuitive trials, the goal is to lose. The drug is tested on recreational opioid users to measure its 'liking' score. Success is defined by demonstrating the new drug is significantly less preferable than existing abusable opioids like Oxycodone.